The quantity of cosmic rays bombarding the earth affects the amount of C that is created in the upper atmosphere. The level of cosmic rays varies with the sun's activity, the strength of the Earth's magnetic field, and any magnetic clouds traversed by the solar system as it proceeds around our galaxy.
Radiocarbon dating
The C dating system assumes that C in the animal or plant matches the level in the general environment. In rare cases, plants and animals may live in very unusual environments whose C content is much lower than what one would expect. This is called a " reservoir effect. It is possible for snails to live in water that contains carbon leached out of ancient limestone which has no measurable C left. As a result, the snails' shells will also be deficient in C and test older than their true age.
Dating advances
In a few areas of the world, seals dine on fish that in turn had eaten other fish and plants that lived in sea water that has been traveling along the bottom of the ocean for thousands of years, gradually losing its C content. Again, the quantity of C in their environment is deficient. They would also test older than they really are. Porous samples can contain recently living material with a full "charge" of C Sample cleaning and proper laboratory technique are critical.
Extending the calibration curve to cover older samples: According to Tom Gidwitz: Living snails were C14 dated at 2, and 27, years old ," EvoWiki. Go to the previous page, or to the "C dating" menu , or choose: About this site About us Our beliefs Your first visit? Quick Links Top Menu. This page translator works on Firefox, Opera, Chrome, and Safari browsers only After translating, click on the "show original" button at the top of this page to restore page to English.
This method is called "insoluble collagen extraction" in this database. Longin showed that collagen could be extracted in a soluble form that permitted a greater degree of decontamination of the sample. Haynes presented a method of extracting the inorganic carbon from bone.
The Limitations of Carbon Dating
This method was considered suitable for use in areas where collagen is rarely or poorly preserved in bones. Subsequent research cast doubt on the reliability of this method. Hassan and others ; Hassan and Ortner, showed that the inorganic carbon contained in bone apatite is highly susceptible to contamination by either younger or older carbon in the burial environment. It now appears that insoluble collagen extractions usually err on the young side, if at all Rutherford and Wittenberg, , whereas bone apatite can produce ages either older or younger than the true age, often by a considerable margin.
Ongoing research has continued to refine methods of extracting collagen, especially from small samples destined for AMS dating. Stafford ; Stafford, et al. Hedges and Van Klinken review other recent advances in the pre-treatment of bone. One of the initial assumptions of the method was that the rate of production of radiocarbon is constant.
This assumption is now known to be incorrect, meaning that radiocarbon years are not equivalent to calendar years. International collaboration by many laboratories has produced increasingly refined calibration curves. The latest calibration dataset, known as INTCAL98, links the dated tree-ring record to the uranium-thorium dating of corals and finally to terrestrial varve chronologies to achieve calibration over the interval , years.
Some studies can be conducted entirely in terms of radiocarbon years. Other studies, such as those focused on rates of change, may require more or less precise calibrations. Land plants and the food chains they support acquire most of their carbon from the atmosphere, whereas marine food chains acquire carbon mainly from the oceans. Upward flow of deep ocean water also brings ancient, non-radioactive carbon to the surface waters. Therefore marine organisms are relatively depleted in C, and modern marine plants and animals can yield apparent ages of hundreds of years.
This discrepancy is called the reservoir effect.
It was once thought that the reservoir effect was about years in all the oceans, but it is now known that the size of the effect varies geographically and through time. Every regional study that employs radiocarbon dates on marine organisms must establish the appropriate correction factor for that region.
Limitations of and extensions to the C dating technique
Hans Suess was the first to point out that the burning of fossil fuels has a profound influence on carbon reservoirs. Indeed some of these materials are used as standards to enable the laboratories to monitor the background radiation. When the fuels are burned, their carbon is released into the atmosphere as carbon dioxide and certain other compounds. During photosynthesis, plants discriminate against the heavier isotopes of carbon, taking up proportionally less C and C than is available in their carbon reservoir.
The result is isotopic fractionation, and it is passed along to the consumers of the plants the herbivores and to their consumers the carnivores.
In fact, additional fractionation occurs when herbivores eat the plants and when carnivores eat the herbivores. It is believed that all organisms discriminate against C about twice as much as against C, and the ratio between the stable C and C atoms can be used to correct for the initial depletion of C Radiocarbon dates can be corrected for isotopic fractionation, a correction called normalization. The amount of isotopic fractionation depends on the photosynthetic pathway used by the plant.
- craigslist dating safety!
- halifax dating scene!
- Radiocarbon Dating Principles.
- attractive professionals dating agency reviews!
Most flowering plants, trees, shrubs and temperate zone grasses are known as C3 plants, because they create a molecule with three carbon atoms using the Calvin-Benson photosynthetic cycle. Grasses that are adapted to arid regions, such as buffalo grass Bouteloua and maize Zea , are known as C4 plants, because they create a molecule with four carbon atoms using the Hatch-Slack cycle. C3 plants discriminate against the heavier carbon isotopes more strongly than do C4 plants.
Normalization is a correction for isotopic fractionation. For example, most C3 plants have C ratios near parts per mil, whereas C ratios in C4 plants are in the range of to Herbivores are less selective against the heavier isotopes, and their bone collagen is enriched by 5 parts per mil in relation to their diet. Yet another change occurs in carnivores whose bone collagen is enriched by an additional 1 part per mil.
What is radiocarbon dating?
Marine plants are similar to C3 plants, but they obtain their carbon from dissolved oceanic bicarbonates that differ from the atmosphere in their isotope ratios, and this difference is passed up the marine food chain. Radiocarbon dates can be normalized to any chosen value, and the value chosen by international convention is parts per mil based on an internationally accepted oak standard. Every part per mil difference from is equivalent to 16 years. For example, bone collagen from marine mammals commonly has a C ratio of parts per mil.
That difference of 10 parts per mil from the oak standard means that the age of the marine mammal bone can be normalized by adding years to its measured age. While the lighter isotopes 12 C and 13 C are stable, the heaviest isotope 14 C radiocarbon is radioactive. This means its nucleus is so large that it is unstable. Over time 14 C decays to nitrogen 14 N.
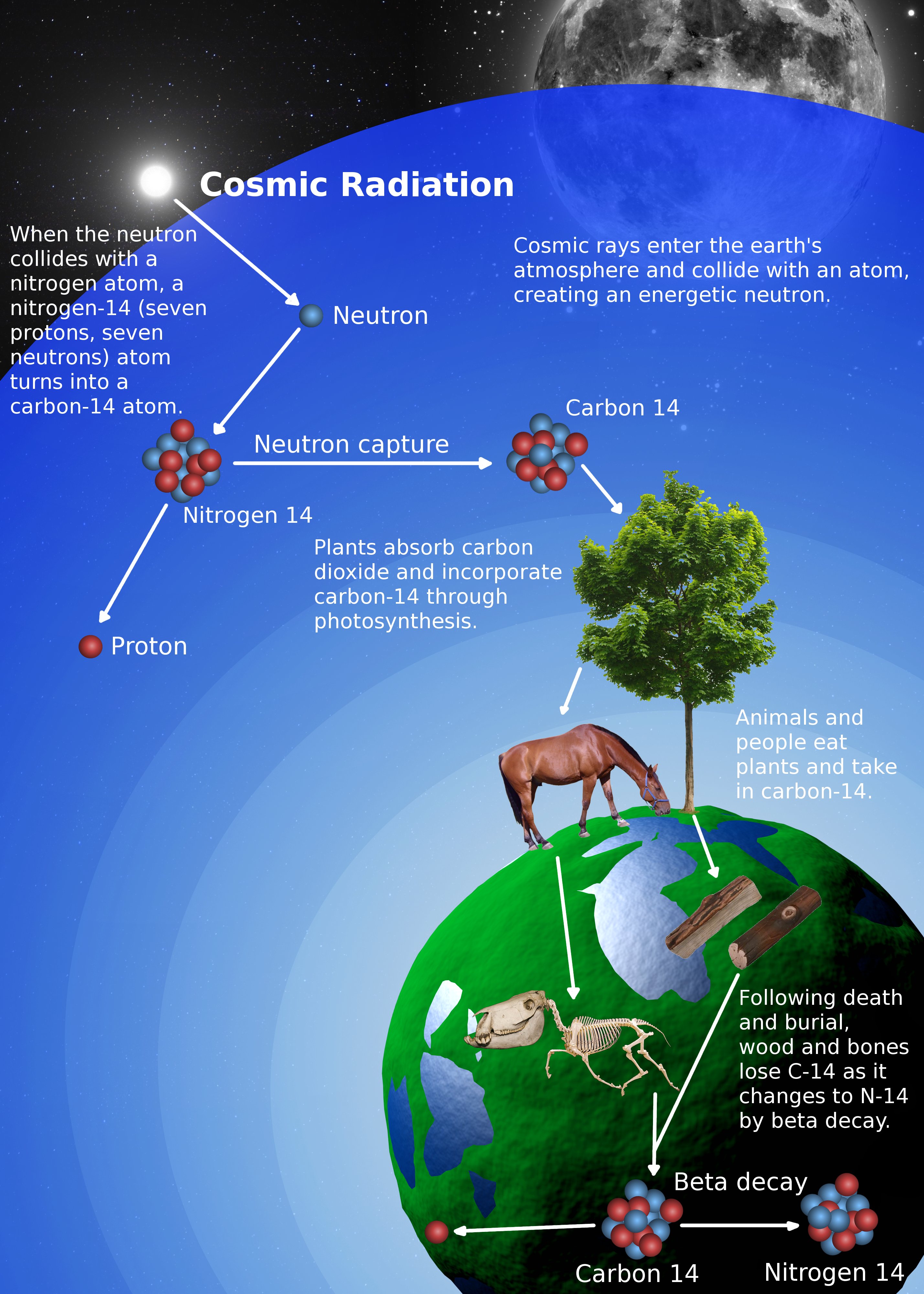
Most 14 C is produced in the upper atmosphere where neutrons, which are produced by cosmic rays , react with 14 N atoms. This CO 2 is used in photosynthesis by plants, and from here is passed through the food chain see figure 1, below. Every plant and animal in this chain including us!
When living things die, tissue is no longer being replaced and the radioactive decay of 14 C becomes apparent. Around 55, years later, so much 14 C has decayed that what remains can no longer be measured. In 5, years half of the 14 C in a sample will decay see figure 1, below. Therefore, if we know the 14 C: Unfortunately, neither are straightforward to determine. The amount of 14 C in the atmosphere, and therefore in plants and animals, has not always been constant. For instance, the amount varies according to how many cosmic rays reach Earth.
Luckily, we can measure these fluctuations in samples that are dated by other methods. Tree rings can be counted and their radiocarbon content measured. A huge amount of work is currently underway to extend and improve the calibration curve.
In we could only calibrate radiocarbon dates until 26, years. Now the curve extends tentatively to 50, years. Radiocarbon dates are presented in two ways because of this complication. The uncalibrated date is given with the unit BP radiocarbon years before The calibrated date is also presented, either in BC or AD or with the unit calBP calibrated before present - before The second difficulty arises from the extremely low abundance of 14 C.
Many labs now use an Accelerator Mass Spectrometer AMS , a machine that can detect and measure the presence of different isotopes, to count the individual 14 C atoms in a sample.