The biggest assumption is that, to first order, the number of asteroids and comets hitting the Earth and the Moon was the same as for Mercury, Venus, and Mars. There is a lot of evidence that this is true. The bottom line is that the more craters one sees, the older the surface is. This can be interpreted in two ways: Based on our study of meteorites and rocks from the Moon, as well as modeling the formation of planets, it is believed pretty much well-established that all of the objects in the Solar System formed very quickly about 4.
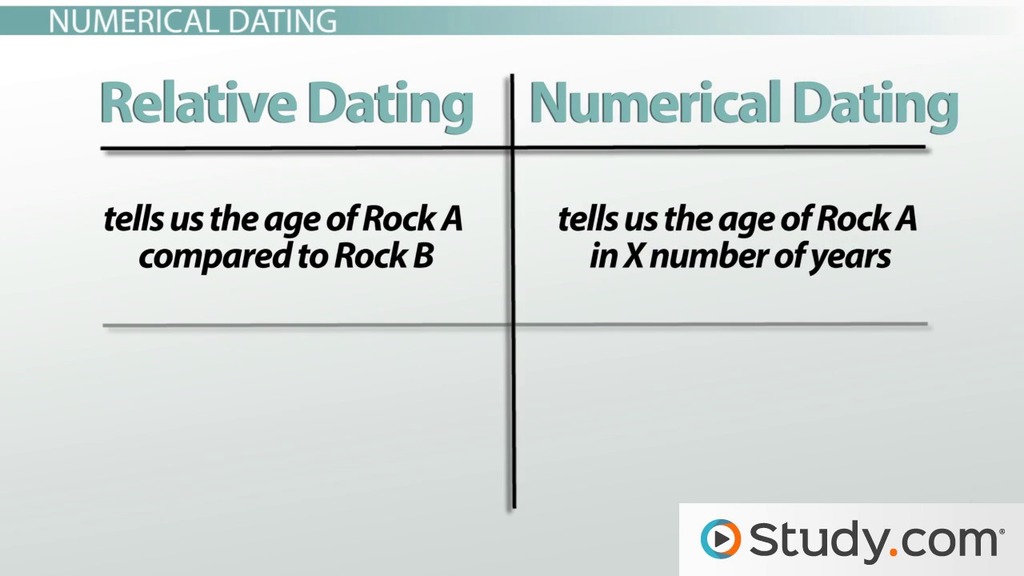
When we age date a planet, we are actually just dating the age of the surface, not the whole planet. We can get absolute ages only if we have rocks from that surface. For others, all we are doing is getting a relative age, using things like the formation of craters and other features on a surface. By studying other planets, we are learning more about our own planet. The effects of impacts and how they might affect us here on Earth, global climate change Venus vs.
Earth and what could happen to Earth in an extreme case, etc. From Wikipedia, radioactive decay is the process in which an unstable atomic nucleus spontaneously loses energy by emitting ionizing particles and radiation.
Radiometric dating
This decay, or loss of energy, results in an atom element of one type, called the parent nuclide transforming to an atom of a different type another element or another isotope of the same element , named the daughter nuclide. It is impossible to predict when a given atom will decay, but given a large number of similar atoms, the decay rate on average is predictable. This predictable decay is called the half-life of the parent atom, the time it takes for one half of all of the parent atoms to transform into the daughter.
This may simply have to do with what the media is talking about.
When there is a scientific discussion about the age of, say a meteorite or the Earth, the media just talks about the large numbers and not about the dating technique e. On the other hand, when the media talk about "more recent events," ages that are more comprehendible, such as when early Man built a fire or even how old a painting is or some ancient parchment , then we bring up the dating technique in order to better validate the findings. Carbon is unreactive with a number of common lab substances: It cannot be used to accurately date a site on its own.
FAQ - Radioactive Age-Dating | Planetary Science Institute
However, it can be used to confirm the antiquity of an item. Optically stimulated luminescence OSL dating constrains the time at which sediment was last exposed to light. During sediment transport, exposure to sunlight 'zeros' the luminescence signal. Upon burial, the sediment accumulates a luminescence signal as natural ambient radiation gradually ionises the mineral grains.
Careful sampling under dark conditions allows the sediment to be exposed to artificial light in the laboratory which releases the OSL signal. The amount of luminescence released is used to calculate the equivalent dose De that the sediment has acquired since deposition, which can be used in combination with the dose rate Dr to calculate the age. Dendrochronology or tree-ring dating is the scientific method of dating based on the analysis of patterns of tree rings , also known as growth rings.
Dendrochronology can date the time at which tree rings were formed, in many types of wood, to the exact calendar year. Dendrochronology has three main areas of application: In some areas of the world, it is possible to date wood back a few thousand years, or even many thousands. Currently, the maximum for fully anchored chronologies is a little over 11, years from present.
Amino acid dating is a dating technique [5] [6] [7] [8] [9] used to estimate the age of a specimen in paleobiology , archaeology , forensic science , taphonomy , sedimentary geology and other fields. This technique relates changes in amino acid molecules to the time elapsed since they were formed. All biological tissues contain amino acids. All amino acids except glycine the simplest one are optically active , having an asymmetric carbon atom.
You are here
This means that the amino acid can have two different configurations, "D" or "L" which are mirror images of each other. With a few important exceptions, living organisms keep all their amino acids in the "L" configuration. When an organism dies, control over the configuration of the amino acids ceases, and the ratio of D to L moves from a value near 0 towards an equilibrium value near 1, a process called racemization. Thus, measuring the ratio of D to L in a sample enables one to estimate how long ago the specimen died.
From Wikipedia, the free encyclopedia. This section does not cite any sources. Please help improve this section by adding citations to reliable sources. Unsourced material may be challenged and removed. July Learn how and when to remove this template message. Archaeology of ancient Mexico and Central America: Down to Earth Fifth edition. American Journal of Archaeology. Annual Review of Earth and Planetary Sciences. When an organism dies, it ceases to take in new carbon, and the existing isotope decays with a characteristic half-life years. The proportion of carbon left when the remains of the organism are examined provides an indication of the time elapsed since its death.
This makes carbon an ideal dating method to date the age of bones or the remains of an organism. The carbon dating limit lies around 58, to 62, years.
- matchmaking agencies reviews?
- online dating going nowhere?
- Relative Dating.
- FAQ - Radioactive Age-Dating?
- free dating sites mississippi?
- Absolute dating?
The rate of creation of carbon appears to be roughly constant, as cross-checks of carbon dating with other dating methods show it gives consistent results. However, local eruptions of volcanoes or other events that give off large amounts of carbon dioxide can reduce local concentrations of carbon and give inaccurate dates. The releases of carbon dioxide into the biosphere as a consequence of industrialization have also depressed the proportion of carbon by a few percent; conversely, the amount of carbon was increased by above-ground nuclear bomb tests that were conducted into the early s.
Also, an increase in the solar wind or the Earth's magnetic field above the current value would depress the amount of carbon created in the atmosphere. This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the spontaneous fission of uranium impurities. The uranium content of the sample has to be known, but that can be determined by placing a plastic film over the polished slice of the material, and bombarding it with slow neutrons.
This causes induced fission of U, as opposed to the spontaneous fission of U. The fission tracks produced by this process are recorded in the plastic film. The uranium content of the material can then be calculated from the number of tracks and the neutron flux. This scheme has application over a wide range of geologic dates. For dates up to a few million years micas , tektites glass fragments from volcanic eruptions , and meteorites are best used.
Older materials can be dated using zircon , apatite , titanite , epidote and garnet which have a variable amount of uranium content.
- hook up telephone line?
- dating a guy 5 years younger than me?
- Absolute Dating.
- Dating Fossils – How Are Fossils Dated??
- psp dating sims?
- western dating rules?
The technique has potential applications for detailing the thermal history of a deposit. The residence time of 36 Cl in the atmosphere is about 1 week. Thus, as an event marker of s water in soil and ground water, 36 Cl is also useful for dating waters less than 50 years before the present. Luminescence dating methods are not radiometric dating methods in that they do not rely on abundances of isotopes to calculate age. Instead, they are a consequence of background radiation on certain minerals.
Over time, ionizing radiation is absorbed by mineral grains in sediments and archaeological materials such as quartz and potassium feldspar. The radiation causes charge to remain within the grains in structurally unstable "electron traps". Exposure to sunlight or heat releases these charges, effectively "bleaching" the sample and resetting the clock to zero. The trapped charge accumulates over time at a rate determined by the amount of background radiation at the location where the sample was buried.
Stimulating these mineral grains using either light optically stimulated luminescence or infrared stimulated luminescence dating or heat thermoluminescence dating causes a luminescence signal to be emitted as the stored unstable electron energy is released, the intensity of which varies depending on the amount of radiation absorbed during burial and specific properties of the mineral.
These methods can be used to date the age of a sediment layer, as layers deposited on top would prevent the grains from being "bleached" and reset by sunlight. Pottery shards can be dated to the last time they experienced significant heat, generally when they were fired in a kiln. Absolute radiometric dating requires a measurable fraction of parent nucleus to remain in the sample rock. For rocks dating back to the beginning of the solar system, this requires extremely long-lived parent isotopes, making measurement of such rocks' exact ages imprecise.
To be able to distinguish the relative ages of rocks from such old material, and to get a better time resolution than that available from long-lived isotopes, short-lived isotopes that are no longer present in the rock can be used.
At the beginning of the solar system, there were several relatively short-lived radionuclides like 26 Al, 60 Fe, 53 Mn, and I present within the solar nebula. These radionuclides—possibly produced by the explosion of a supernova—are extinct today, but their decay products can be detected in very old material, such as that which constitutes meteorites. By measuring the decay products of extinct radionuclides with a mass spectrometer and using isochronplots, it is possible to determine relative ages of different events in the early history of the solar system.
Dating methods based on extinct radionuclides can also be calibrated with the U-Pb method to give absolute ages. Thus both the approximate age and a high time resolution can be obtained. Generally a shorter half-life leads to a higher time resolution at the expense of timescale. The iodine-xenon chronometer [32] is an isochron technique.
Navigation menu
Samples are exposed to neutrons in a nuclear reactor. This converts the only stable isotope of iodine I into Xe via neutron capture followed by beta decay of I. After irradiation, samples are heated in a series of steps and the xenon isotopic signature of the gas evolved in each step is analysed. Samples of a meteorite called Shallowater are usually included in the irradiation to monitor the conversion efficiency from I to Xe. This in turn corresponds to a difference in age of closure in the early solar system. Another example of short-lived extinct radionuclide dating is the 26 Al — 26 Mg chronometer, which can be used to estimate the relative ages of chondrules.
The 26 Al — 26 Mg chronometer gives an estimate of the time period for formation of primitive meteorites of only a few million years 1. From Wikipedia, the free encyclopedia. Earth sciences portal Geophysics portal Physics portal. The disintegration products of uranium". American Journal of Science. Radiometric Dating and the Geological Time Scale: Circular Reasoning or Reliable Tools?